AstraZeneca has received the nod from the Subject Expert Committee (SEC) of the Central Drugs Standard Control Organization (CDSCO) to conduct a Phase III clinical trial of anti-cancer drug Durvalumab.
The trial will evaluate the efficacy and safety of Durvalumab in combination with chemotherapy in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have progressed after platinum-based therapy.
Durvalumab is an immune checkpoint inhibitor that works by blocking the PD-1 protein on T cells. PD-1 is a checkpoint that helps to keep the immune system from attacking healthy cells. By blocking PD-1, Durvalumab helps to unleash the immune system’s attack on cancer cells.
The Phase III trial is expected to enroll around 700 patients in India. The trial will be conducted at over 100 clinical sites across the country.
The results of the trial are expected to be available in 2024. If the trial is successful, Durvalumab could become a new treatment option for patients with NSCLC.


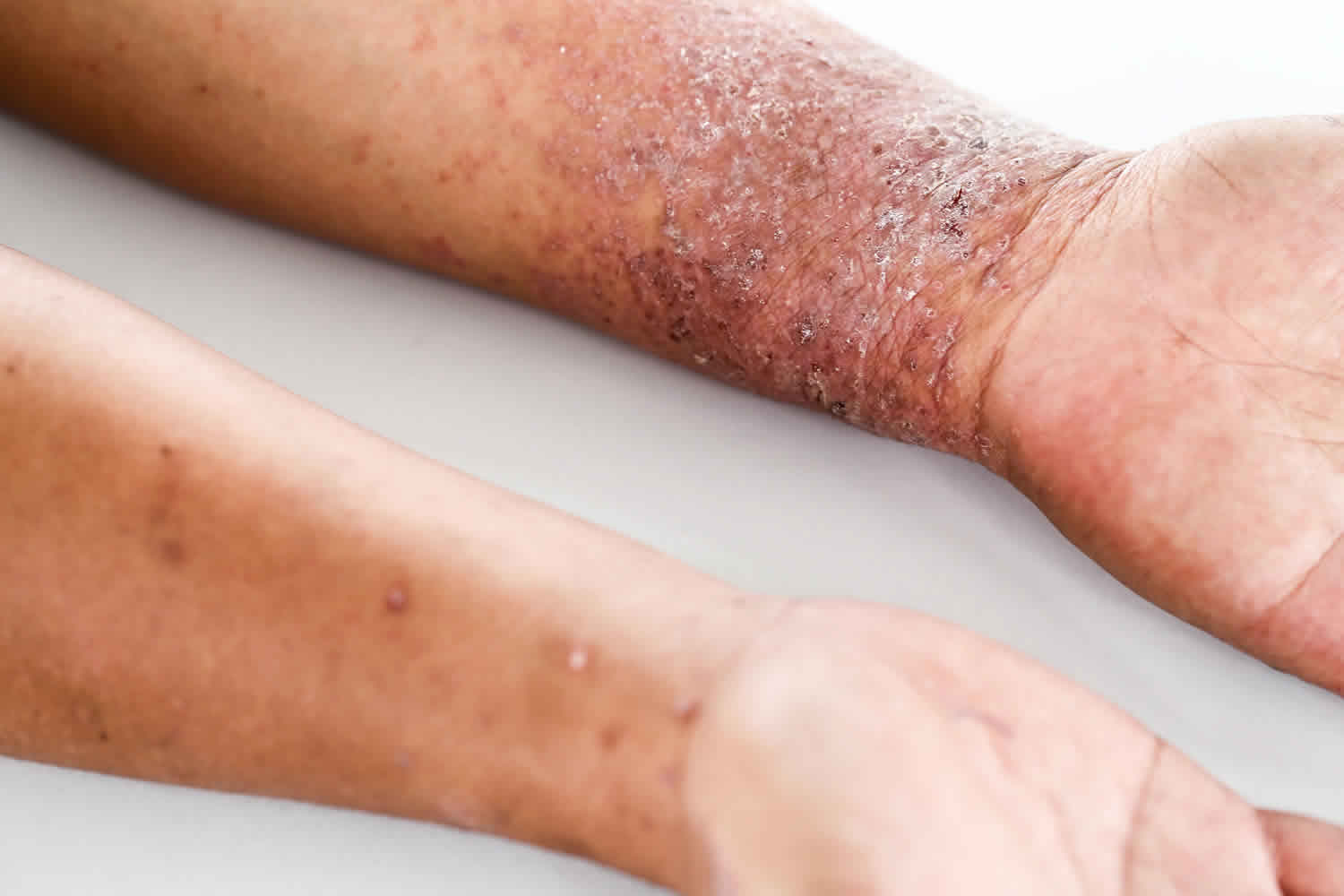


Morbi lectus risus, iaculis vel, suscipit quis, luctus non, massa. Fusce ac turpis quis ligula lacinia aliquet. Mauris ipsum. Nulla metus metus, ullamcorper vel, tincidunt sed, euismod in, nibh.