Sanofi Healthcare India Pvt. Ltd. has received marketing approval for Dupixent (dupilumab), a medicine used to treat moderate-to-severe atopic dermatitis in adults and children aged 6 years and older. This is great news for patients in India who suffer from this condition.
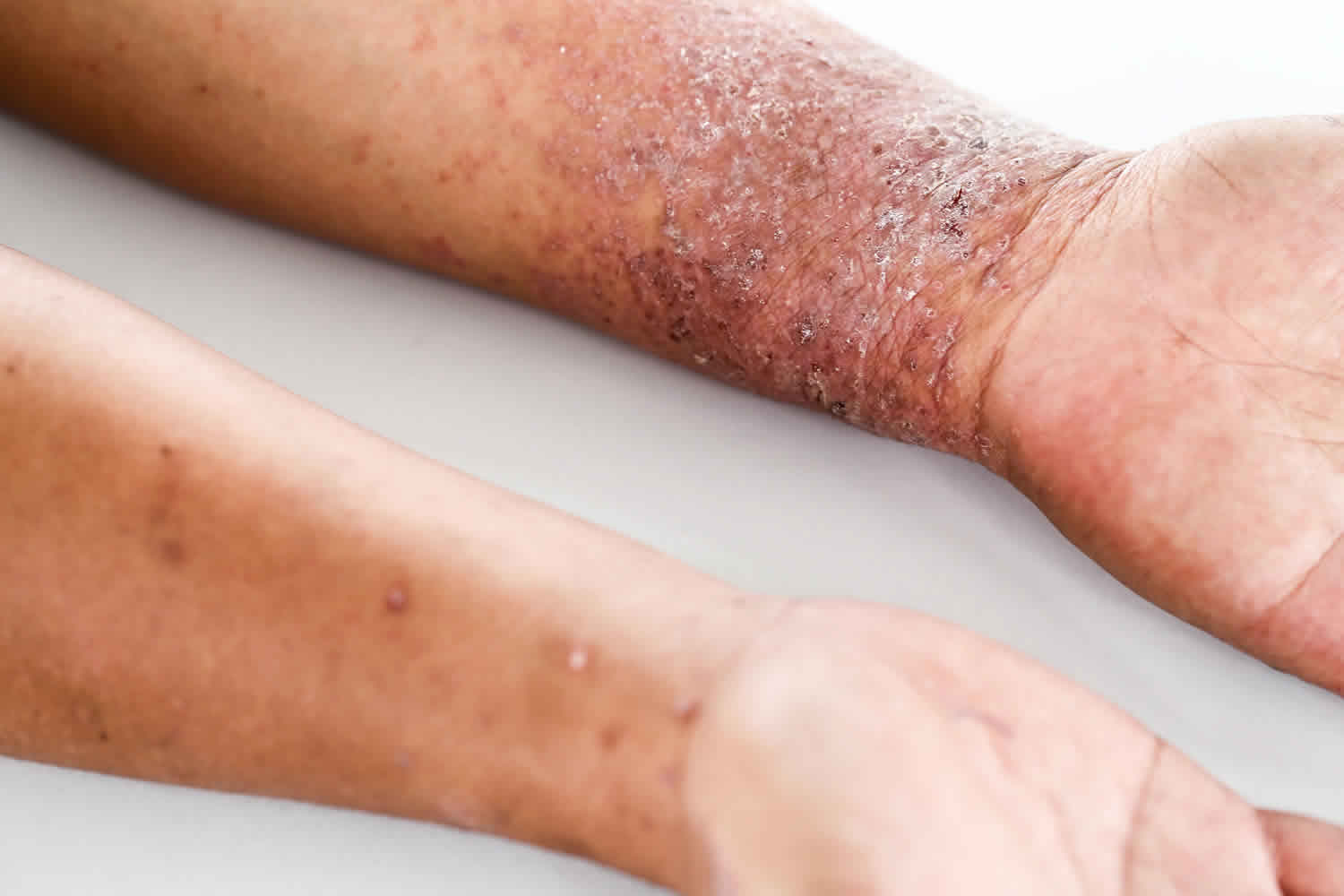
Atopic dermatitis, also known as eczema, is a chronic skin condition that causes inflammation, itching, and dryness of the skin. It is quite common, affecting up to 20% of children and 1% of adults worldwide. People with a family history of atopic dermatitis or other allergic conditions are more likely to develop this condition.
While there is no cure for atopic dermatitis, there are treatments available to manage its symptoms. These treatments include topical steroids, oral medications, and biologic therapies like Dupixent.
Dupixent is a biologic therapy that is injected under the skin once every two weeks. It works by blocking the activity of two proteins involved in the inflammation that causes atopic dermatitis. Clinical studies have shown that Dupixent significantly improves the signs, symptoms, and quality of life in patients with moderate-to-severe atopic dermatitis.
The approval of Dupixent in India is a significant milestone for patients with atopic dermatitis. It provides them with a new and effective treatment option, especially for those who have not responded well to other treatments. Dupixent has been proven to reduce itching, improve skin dryness, and enhance sleep quality in patients. It also helps reduce the risk of flares in those with atopic dermatitis.
Patients can look forward to the availability of Dupixent in India in the coming months. This approval brings hope and relief to individuals struggling with the symptoms of atopic dermatitis, giving them a chance for a better quality of life.